

Bohr Model of All Elementsīohr diagrams of all elements are shown in the table below. Niels Bohr introduced the atomic Hydrogen model in 1913. Thus, 1st shell can hold 2 electrons, 2nd shell can hold 8 electrons, 3rd shell can hold 18 electrons, 4th shell can hold 32 electrons, and so on. of electrons this orbit can hold K shell, n = 1 2 L shell, n = 2 8 M shell, n = 3 18 N shell, n = 4 32. Number of electrons in each shell Orbit / Shell (n) Maximum no. Here is a simple table showing the number of electrons that can be accommodated in each shell. All features of Bohr’s model of the atom can be summarized in Bohr’s Postulates. Bohr’s model of the hydrogen atom, proposed by Niels Bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. The electrons move in a fixed orbits (shells) and each orbit has a fixed energy.Įach orbit (or shell) can hold a certain number of electrons. In atomic physics, the Bohr model of the atom (also known as the Rutherford-Bohr model) is the modern model of the hydrogen atom introduced by Danish physicist Niels Bohr. In the Bohr model of atomic structure, electrons are constrained to orbit a nucleus at specific distances, given by the equation where r is the radius of the orbit, Z is the charge on the nucleus, a0 is the Bohr radius and has a value of 5.292 10-11 m, and n is a positive integer (n 1, 2, 3.) like a principal quantum number. This is similar to the planets revolving around the sun, except that the orbits are non-planar. In Bohrs model of the hydrogen atom, it is a system consisting of a dense positively charged nucleus surrounded by orbiting electrons. Web The electrons speed is largest in the first Bohr orbit for n 1 which is the orbit closest to.Īn important criterion for the validity of a quantum mechanical.Bohr model is a structural model in which the negatively charged electrons revolve around the positively charged nucleus. And even though this is not reality, the Bohr model is not exactly what's happening, it is a useful model to think about. In 1913, after returning to Copenhagen, he began publishing his theory of the simplest atom, hydrogen, based on the. The number of times larger the spacing between the energy levels with n 3 and n 8 spacing between the energy level with n 8 and n 9 for the hydrogen atom is 3.23. We went over the Bohr model of the hydrogen atom, which has one proton in its nucleus, so here's the positive charge in the nucleus, and a negatively charged electron orbiting the nucleus. The concept was promoted by quantum physicist Niels Bohr c. In the model, electrons orbitorbitA fixed orbit is the concept, in atomic physics, where an electron is considered to remain in a specific orbit, at a fixed distance from an atom's nucleus, for a particular energy level. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud.
Bohr became convinced of its validity and spent part of 1912 at Rutherford’s laboratory. Niels Bohr - Wikipedia introduced the atomic Hydrogen model in 1913. Web Bohr model energy levels Google Classroom About Transcript Calculating electron. The great Danish physicist Niels Bohr (18851962) made immediate use of Rutherford’s planetary model of the atom. Web Bohrs theory tells us that the ionisation energy for one-electron atoms. Web The electrons speed is largest in the first Bohr orbit for n 1 which is the. Web Bohrs model equation is E2 - E1 h x f f E2 - E1h f 24 - 1566261 x 10 -34. Web Bohrs Model Calculator To determine the frequency of the electromagnetic wave. Web The Bohr radius a 0 is a physical constant approximately equal to the most probable. Web The Bohr Model Calculator allows you to determine the energy difference between an. Web The solar system or planetary model of the atom was attractive to. Web which is identical to the Rydberg equation in which R k h c.
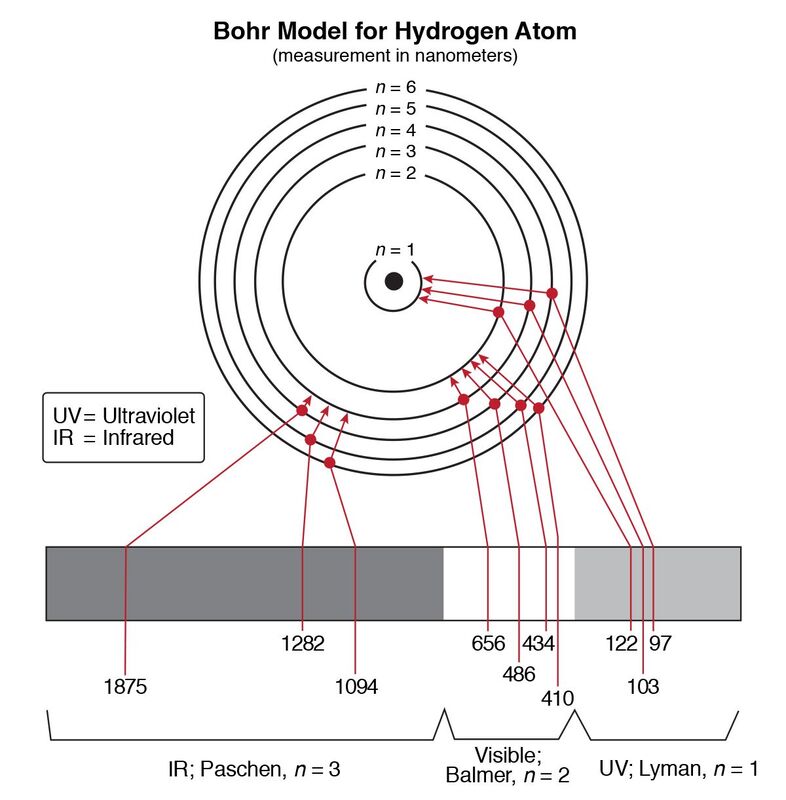
Hydrogen Energies And Spectrum Web This formula will work for hydrogen and other unielecton ions like He Li2 etc. Web Using the Bohr model determine the energy in joules of the photon produced when an. Using the Bohr model calculate the energy associated with the. The Bohr model is used to account for the spectrum of the hydrogen atom, but the basic idea is the same for all elements.


 0 kommentar(er)
0 kommentar(er)
